Parkinson’s disease (PD) is the second most common neurodegenerative disease, affecting more than ten million people worldwide. There are 90,000 people newly diagnosed every year in the U.S. alone.
Even with current standard of care therapy, patients can eventually develop debilitating motor complications. The economic burden of disease is estimated to be over $51 billion annually (MJFF, 2019)
Currently, there are no disease-modifying agents available to patients.

Regenerative Therapy for PD
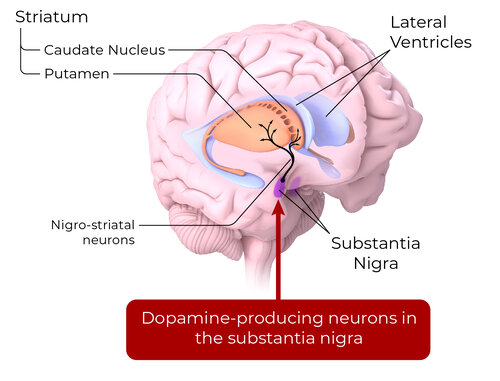
Around 50% of dopamine neurons in the substantia nigra are lost before a PD diagnosis. Cell replacement therapy of dopamine neurons has the potential to release dopamine and reconstruct neural networks
Autologous dopamine neurons are an individualized approach to cell replacement therapy.
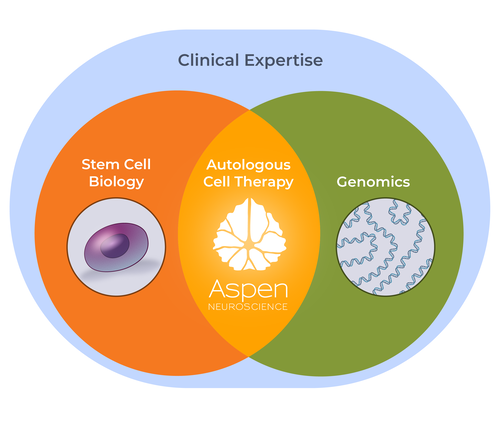
Leveraging Stem Cell Biology & Genomics
Aspen Neuroscience is the leading company using a patient’s own cells to develop cell replacement therapies.
Induced Pluripotent Stem Cells (iPSCs) are derived from somatic cells by transient expression of pluripotency-associated transcription factors (Yamanaka Patents).
The company has developed a best-in-class platform to create and optimize pluripotent-derived cell therapies, which includes in-house bioinformatics, manufacturing and quality control. Aspen combines cell biology with the latest machine learning and genomic approaches to investigate patient-specific, restorative cell treatments.
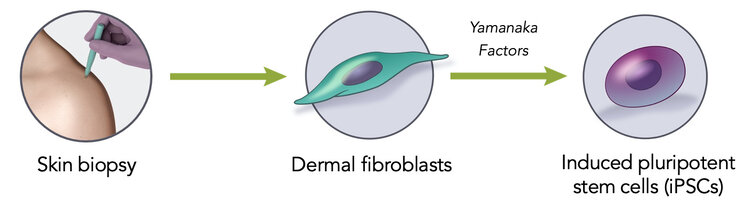
Our manufacturing process starts from a small sample of the patient’s own skin cells, followed by reprogramming to induced pluripotent stem cells (iPSCs) and then differentiation of the iPSCs into DANPCs. These DANPCs are transplanted into the putamen, replacing cells that were lost or damaged due to disease. The quality of each person’s cells is assessed at every manufacturing stage using Aspen’s proprietary machine learning-based genomics tests.
Potential Benefits of Autologous Therapy
We are scaling out manufacturing in preparation to provide a personalized cell therapy that avoids the need for immunosuppression.
- A personalized cell therapy can be developed from a small skin biopsy
- Utilizing autologous cell therapies can avoid the need for immunosuppression