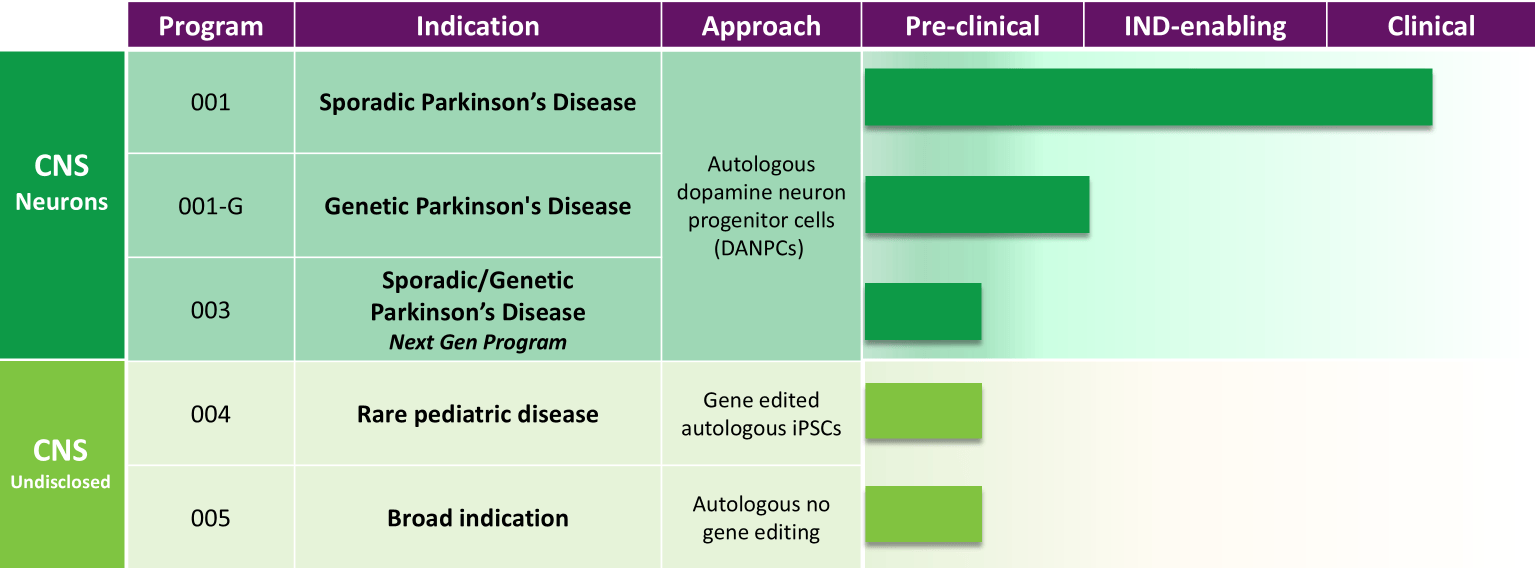
Our clinical development programs encompass multiple candidates for cell replacement therapy within the Central Nervous System (CNS).
We are also investigating several candidates for the treatment of both sporadic and genetic forms of Parkinson’s disease, as well as additional candidates targeted in the CNS.