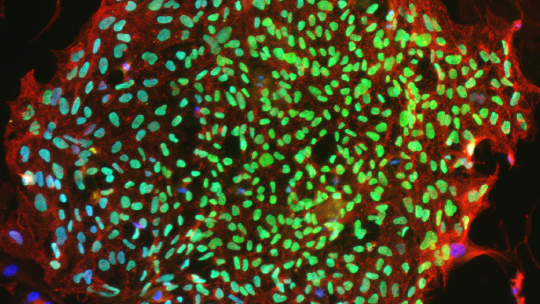
Aspen Neuroscience to Present at American Society of Gene & Cell Therapy (ASGCT) Annual Meeting
Presentation Covers Aspen’s Autologous iPSC-derived Approach for Neuron Replacement Therapy in Trial for Parkinson’s Disease SAN DIEGO, May 14, 2025 /PRNewswire/ — Aspen Neuroscience Chief Scientific Officer Xiaokui Zhang, Ph.D., will present at the American Society of Gene & Cell Therapy (ASGCT) 28th Annual Meeting, held this week May 13-17, at the Ernest N. Morial Convention Center in New Orleans, Louisiana. The annual … Read more
Aspen Neuroscience does not make determination on patient eligibility for clinical trials and all trial related enrollment and eligibility decisions are made by clinical trial sites per protocol under the direction of a Principal Investigator. ANPD001 is investigational and is not yet approved by the FDA. At this time, Aspen Neuroscience does not offer or participate in compassionate use or expanded access programs for ANPD001.