Parkinson’s disease (PD) is the second most common neurodegenerative disease, affecting more than ten million people worldwide. There are 60,000 people newly diagnosed every year.
Even with the current standard of care therapy, patients can eventually develop debilitating motor complications. The economic burden of disease is estimated to be over $51 billion annually (MJFF, 2019)

Currently, there are no disease-modifying agents available to patients.
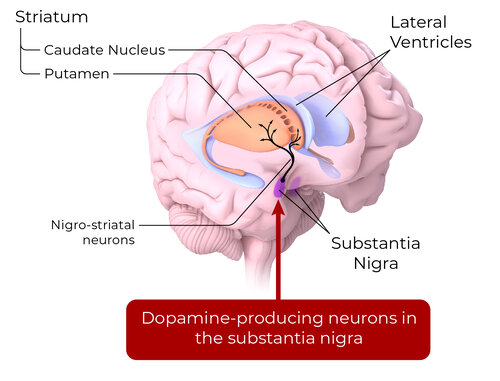
REGENERATIVE THERAPY FOR PD
Around 50% of dopamine neurons in the substantia nigra are lost before a PD diagnosis. Cell replacement therapy of dopamine neurons has the potential to release dopamine and reconstruct neural networks
Stem cell-derived dopamine neurons are an individualized disease-modifying approach for restorative cell replacement therapy.
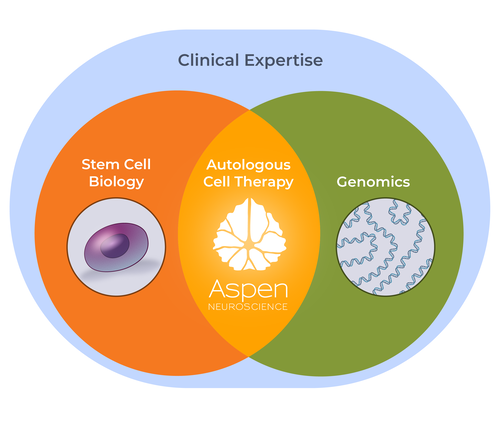
LEVERAGING STEM CELL BIOLOGY & GENOMICS
Aspen Neuroscience is the leading company using a patient’s own cells to develop cell replacement therapies.
Induced Pluripotent Stem Cells (iPSCs) are derived from somatic cells by transient expression of pluripotency-associated transcription factors (Yamanaka Patents).
iPSCs are functionally indistinguishable from embryonic stem cells derived from 5-day embryos.
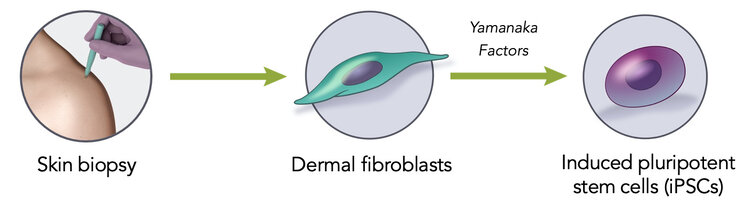
iPSCs have all the strengths of embryonic stem cells without the limitations of allogeneic therapy.
Comparison of Autologous and Allogeneic Therapy
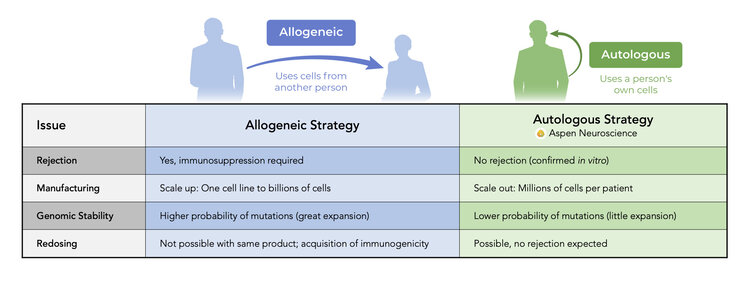
We are scaling out manufacturing in preparation to provide a personalized cell therapy that avoids the need for immunosuppression.